
CRISPR and Beyond: The Future of Genetic Engineering.
Exploring the revolutionary impact of CRISPR-Cas9 on genetic engineering, this article delves into how gene-editing technology is transforming medicine, agriculture, and biotechnology. It highlights current applications, limitations, and emerging advancements like base editing and prime editing, while addressing ethical and regulatory challenges. Looking beyond CRISPR, the future of genetic engineering promises unprecedented precision and innovation, poised to reshape healthcare, food security.
✨ Raghav Jain
Introduction
Genetic engineering has revolutionized biology and medicine, offering unprecedented control over the fundamental blueprint of life — DNA. From the earliest recombinant DNA technologies in the 1970s to today’s powerful gene-editing tools, humanity has progressively unlocked the ability to modify genes with precision and intention. At the forefront of this revolution stands CRISPR-Cas9, a transformative technology that has dramatically accelerated genetic manipulation’s scope, precision, and affordability.
However, CRISPR is not the end of the story. As science pushes beyond CRISPR, new tools and technologies promise to reshape genetics, biotechnology, agriculture, and medicine in even more profound ways. This article explores the origins of CRISPR, its current applications, the limitations it faces, and the exciting future that lies beyond it.
What is CRISPR?
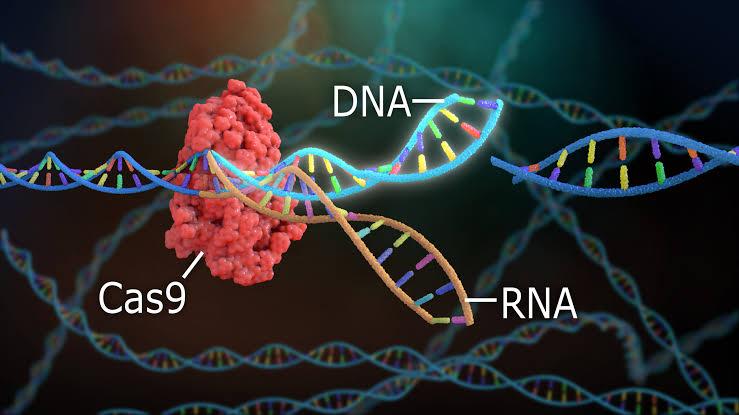
CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats. It is a natural defense mechanism found in bacteria and archaea, which use it to recognize and destroy invading viral DNA. Scientists adapted this system into a powerful gene-editing tool by combining the CRISPR sequence with the Cas9 enzyme, which acts as molecular scissors to cut DNA at targeted locations.
By designing a guide RNA sequence complementary to a gene of interest, researchers can direct Cas9 to specific sites in the genome and induce precise cuts. The cell's natural repair mechanisms then either disable the gene or allow the insertion of new genetic material, enabling a wide variety of genetic modifications.
The Impact of CRISPR on Genetic Engineering
CRISPR has made gene editing:
- Highly precise: It can target exact locations in the genome.
- Relatively easy and inexpensive: Compared to previous methods like TALENs or ZFNs, CRISPR requires less specialized equipment and training.
- Versatile: It can be applied across different organisms, including plants, animals, and humans.
- Rapidly deployable: Its simplicity accelerates research timelines dramatically.
These advantages have led to breakthroughs in:
Medicine
- Gene therapy: Treating genetic disorders like sickle cell anemia, cystic fibrosis, and muscular dystrophy.
- Cancer treatment: Engineering immune cells (CAR-T therapy) to target tumors.
- Infectious diseases: Developing antiviral strategies and diagnostic tools.
Agriculture
- Developing drought-resistant, pest-resistant, and nutrient-enriched crops.
- Reducing reliance on chemical pesticides and fertilizers.
- Improving livestock traits for better yield and disease resistance.
Basic Research
- Accelerating functional genomics studies.
- Creating genetically modified organisms (GMOs) to study diseases.
Limitations and Challenges of CRISPR
Despite its revolutionary impact, CRISPR technology has limitations:
Off-target Effects
CRISPR-Cas9 can sometimes cut unintended regions of DNA, potentially causing harmful mutations. Though off-target activity has been reduced through improvements, it remains a concern, especially for clinical applications.
Delivery Challenges
Effectively delivering the CRISPR components to target cells or tissues—particularly in humans—is complex. Viral vectors, lipid nanoparticles, and physical methods each have pros and cons regarding efficiency, safety, and immune response.
Ethical and Regulatory Concerns
Editing human germline cells (embryos) raises profound ethical questions about designer babies, consent, and unintended consequences for future generations. Regulatory frameworks vary globally, influencing research and clinical use.
Limited Editing Types
CRISPR primarily induces DNA cuts, which rely on cellular repair processes that can be unpredictable. Inserting large sequences or making base-level changes can be inefficient or imprecise.
Beyond CRISPR: Emerging Genetic Engineering Technologies
To overcome CRISPR’s limitations and expand its capabilities, researchers are developing next-generation tools and complementary technologies.
1. Base Editing
Base editors enable direct conversion of one DNA base to another without cutting both strands of DNA. This minimizes unwanted mutations and improves precision for correcting point mutations that cause many genetic diseases.
Example: Changing a cytosine (C) to thymine (T) at a precise position can correct a disease-causing mutation in a single step.
2. Prime Editing
Prime editing combines a catalytically impaired Cas9 with a reverse transcriptase enzyme and a specialized guide RNA. It can insert, delete, or replace DNA sequences without double-strand breaks or donor DNA templates.
This method offers greater versatility and fewer off-target effects than traditional CRISPR-Cas9 editing.
3. CRISPR Variants and Alternatives
- Cas12 and Cas13: Target DNA and RNA, respectively, offering new opportunities for gene and transcriptome editing.
- Cas14 and other miniaturized Cas enzymes: Smaller enzymes facilitate delivery into cells.
- RNA editing: Modulating RNA sequences transiently without altering the genome.
4. Epigenome Editing
Rather than changing the DNA sequence, epigenome editing modifies chemical tags that regulate gene expression. This enables reversible control of genes without permanent genetic alterations.
Applications include reprogramming cell fate and treating diseases linked to epigenetic dysregulation.
5. Synthetic Biology and Gene Circuits
Advanced genetic engineering is integrating synthetic biology to design programmable gene circuits within cells. These synthetic systems can respond dynamically to environmental signals or disease states, enabling sophisticated therapies and biosensors.
The Future Landscape of Genetic Engineering
Precision Medicine
The convergence of genetic editing with personalized medicine will allow therapies tailored to individual genetic profiles. Patients could receive gene corrections optimized for their unique mutations, improving outcomes and reducing side effects.
Agricultural Sustainability
Next-gen editing tools will create crops and livestock with optimized traits to address climate change, food security, and environmental impact. For example, editing photosynthesis pathways or nitrogen fixation genes could drastically improve crop yields.
Synthetic Genomes and Xenobiology
Researchers are pushing towards building synthetic genomes from scratch, designing organisms with novel functions or synthetic genetic codes. These efforts could create new materials, biofuels, or even entirely synthetic life forms.
Ethical and Social Considerations
The ease of genetic manipulation necessitates robust ethical frameworks and global regulations. Public engagement and transparency will be critical in addressing concerns over equity, safety, and unintended consequences.
Genetic engineering has undergone an extraordinary transformation over the past few decades, culminating in the discovery and widespread adoption of the revolutionary CRISPR-Cas9 technology, which has fundamentally altered the landscape of molecular biology and biomedical research by offering an unprecedentedly precise, efficient, and cost-effective method for editing DNA, the fundamental blueprint of life; this technology, originally derived from a natural bacterial immune system used to fend off viral invaders, harnesses a guide RNA molecule that directs the Cas9 enzyme to a specific genetic sequence within an organism’s genome, where Cas9 acts as molecular scissors to create a double-strand break, enabling scientists to delete, insert, or modify genes with remarkable accuracy—a feat that was previously difficult, time-consuming, and expensive with earlier gene-editing methods such as zinc finger nucleases or TALENs. The impact of CRISPR has been nothing short of transformative, propelling forward advancements in diverse fields such as medicine, agriculture, and basic research. In medicine, CRISPR has catalyzed the development of innovative gene therapies aimed at curing previously untreatable genetic disorders, including sickle cell anemia and muscular dystrophy, by correcting disease-causing mutations at their source, while also enhancing cancer treatments through the engineering of immune cells that more effectively target tumors, exemplified by CAR-T cell therapies. Moreover, CRISPR-based diagnostics have accelerated the detection of infectious diseases, including rapid and sensitive tests for viral pathogens, which are critical in managing outbreaks and pandemics. Meanwhile, agricultural biotechnology has benefited immensely from CRISPR’s precision, enabling the creation of crop varieties that are more resilient to drought, pests, and environmental stresses, as well as improving nutritional content, thus offering promising solutions to global food security challenges and reducing dependence on chemical pesticides and fertilizers. Despite these profound advantages, CRISPR technology is not without its challenges and limitations; foremost among these are off-target effects, where unintended genomic sites are accidentally edited, potentially leading to deleterious mutations and unforeseen consequences, which necessitates rigorous validation and refinement of CRISPR components to enhance specificity and safety. Furthermore, delivering the CRISPR machinery efficiently and safely into target cells or tissues, particularly in humans, remains a significant technical hurdle, as various delivery vehicles such as viral vectors, lipid nanoparticles, and physical methods each present unique benefits and risks, including immune responses and limited tissue specificity. Ethical considerations also loom large in the application of CRISPR, especially regarding germline editing, which involves making heritable changes to embryos or reproductive cells; the prospect of “designer babies” raises profound moral, social, and regulatory questions about consent, equity, and long-term impacts on the human gene pool, prompting calls for cautious governance and international consensus. To overcome CRISPR’s inherent constraints and expand the toolkit of genetic engineering, researchers have developed next-generation technologies such as base editing, which enables the conversion of one DNA base to another without introducing double-strand breaks, thereby reducing off-target mutations and improving editing precision, particularly for correcting single-nucleotide polymorphisms responsible for many genetic diseases. Another groundbreaking innovation is prime editing, a versatile method combining a catalytically impaired Cas9 fused to a reverse transcriptase enzyme that can insert, delete, or replace DNA sequences with high fidelity and fewer undesired edits, offering broader editing capabilities than traditional CRISPR-Cas9. Additionally, alternative CRISPR systems such as Cas12 and Cas13 target DNA and RNA respectively, opening new avenues for transient genetic modifications and RNA therapeutics without permanent changes to the genome. Epigenome editing represents a parallel frontier, where chemical tags that regulate gene expression are precisely modified without altering the underlying DNA sequence, enabling reversible and tunable control of gene activity that holds promise for treating diseases linked to epigenetic dysregulation, including certain cancers and neurological disorders. Synthetic biology complements these advancements by engineering programmable gene circuits within cells that can respond dynamically to environmental or pathological signals, thereby enabling sophisticated therapeutic interventions and biosensing applications. Looking forward, the future of genetic engineering is poised to profoundly reshape medicine through precision therapies tailored to individual genetic profiles, agriculture through climate-resilient and highly productive crops, and biotechnology through the creation of synthetic organisms with novel capabilities, such as biofactories for sustainable production of fuels, materials, and pharmaceuticals. However, realizing this potential requires addressing ethical, social, and regulatory challenges by fostering transparent public engagement, establishing robust oversight frameworks, and promoting equitable access to these powerful technologies. In summary, while CRISPR-Cas9 has marked a paradigm shift in genetic engineering by making gene editing accessible and efficient, emerging technologies like base editing, prime editing, epigenome editing, and synthetic biology are expanding the horizons of what is possible, offering unprecedented precision, versatility, and control over the genetic code. These innovations herald a new era where genetic engineering can solve some of humanity’s most pressing problems, from curing genetic diseases to securing sustainable food supplies and advancing synthetic life forms, yet their development must proceed responsibly to maximize benefits while minimizing risks and ethical dilemmas, ultimately shaping a future where the promise of genetic engineering is fully realized in a safe, just, and ethical manner.
Genetic engineering, a field that has evolved dramatically since the advent of recombinant DNA technology in the late 20th century, stands today at the precipice of a new era defined by the revolutionary gene-editing tool known as CRISPR-Cas9, a system originally discovered as a bacterial immune mechanism that scientists have adapted into a highly precise, efficient, and relatively simple method for altering the genomes of virtually any organism, from plants and animals to humans, with profound implications across medicine, agriculture, and biotechnology; the CRISPR system works by utilizing a guide RNA molecule designed to target a specific DNA sequence, directing the Cas9 enzyme to create a double-strand break at the chosen location in the genome, which the cell then repairs using endogenous mechanisms, enabling targeted modifications such as gene disruptions, corrections, or insertions, thereby providing unprecedented control over genetic material; the impact of CRISPR has been far-reaching, enabling breakthroughs such as correcting mutations responsible for inherited diseases like sickle cell anemia and cystic fibrosis, engineering immune cells to better fight cancers through CAR-T therapies, and creating genetically modified crops that are more resilient to drought, pests, and environmental stress, all while accelerating research by simplifying the generation of animal models and functional genomics studies; however, despite these successes, CRISPR technology faces challenges, including off-target effects where unintended genomic sites may be cut, raising safety concerns particularly in clinical applications, as well as difficulties in delivering the CRISPR components effectively and safely to the target cells in living organisms, especially humans, necessitating ongoing research into delivery vectors such as viral particles, lipid nanoparticles, and novel physical methods; furthermore, ethical considerations loom large, especially around germline editing which involves heritable changes to embryos and raises debates about “designer babies,” consent from future generations, and the potential socio-economic divides that could arise from unequal access to gene editing technologies, thus demanding robust regulatory frameworks and international cooperation; to overcome limitations inherent to CRISPR-Cas9, scientists have developed advanced gene-editing tools like base editors, which can convert one DNA base into another without inducing double-stranded breaks, thereby reducing unintended mutations and allowing precise correction of point mutations responsible for many genetic disorders, and prime editors, which expand editing capabilities further by combining a modified Cas9 with reverse transcriptase to insert, delete, or replace DNA sequences with high fidelity and versatility; alongside these, alternative CRISPR systems such as Cas12 and Cas13 enzymes offer DNA and RNA editing respectively, expanding the genetic engineering toolbox into realms like transient RNA editing for potentially reversible therapies, and epigenome editing techniques provide means to modify gene expression by altering chemical tags on DNA or histones without changing the underlying sequence, opening avenues for reversible, tunable gene regulation that may address diseases rooted in epigenetic dysfunction; in parallel, synthetic biology integrates gene editing with engineering principles to design programmable gene circuits that enable cells to sense and respond to environmental signals, creating smart therapies and biosensors that could revolutionize medicine and environmental monitoring; looking ahead, the future of genetic engineering promises to usher in a new paradigm of precision medicine where treatments are tailored to individual genomes, improving efficacy and reducing side effects, as well as transformative advances in agriculture through the development of crops capable of thriving amid climate change and producing higher yields with fewer inputs, thereby enhancing food security and sustainability; additionally, the creation of synthetic genomes and novel life forms through genome synthesis may enable production of new materials, biofuels, and pharmaceuticals, pushing the boundaries of what biology can achieve; however, the immense power of these technologies necessitates careful stewardship to navigate the ethical, social, and regulatory challenges they pose, including considerations of biosafety, biosecurity, equitable access, and the potential ecological impacts of releasing genetically modified organisms into the environment; ultimately, while CRISPR-Cas9 has already revolutionized genetic engineering by democratizing genome editing, the development of next-generation technologies such as base and prime editing, epigenome modification, and synthetic biology platforms will expand the scope and precision of genetic interventions, offering unprecedented opportunities to tackle some of the most pressing challenges in health, agriculture, and beyond, provided that these innovations are pursued responsibly, transparently, and inclusively, ensuring that their benefits are accessible to all and their risks carefully managed; thus, as humanity stands on the threshold of a new age in biological sciences, the fusion of these advanced tools holds the promise of not only curing genetic diseases and enhancing food security but also of fundamentally reshaping our relationship with life itself, heralding a future in which the code of life can be written and rewritten with precision and purpose, transforming medicine, industry, and ecosystems alike while challenging society to thoughtfully consider the profound implications of wielding such transformative power.
Conclusion
CRISPR-Cas9 has revolutionized genetic engineering by providing a simple, efficient, and affordable means of editing genomes. Its impact spans medicine, agriculture, and basic research, offering solutions to some of humanity's greatest challenges.
However, CRISPR has its limitations, including off-target effects, delivery issues, and ethical considerations. To address these, innovative technologies such as base editing, prime editing, epigenome editing, and synthetic biology are emerging, promising greater precision, versatility, and control over genetic material.
The future of genetic engineering lies in these advancements, which hold the potential to transform medicine into a truly personalized discipline, sustainably revolutionize agriculture, and unlock new frontiers in synthetic biology.
Nevertheless, these scientific opportunities must be pursued with careful ethical consideration and regulatory oversight to ensure benefits are maximized while risks are minimized. The next decades in genetic engineering promise to be as exciting as they are transformative.
Q&A Section
Q1: What is CRISPR and how does it work?
Ans: CRISPR is a gene-editing technology derived from a natural bacterial defense system. It uses a guide RNA to direct the Cas9 enzyme to a specific DNA sequence, where Cas9 creates a cut. The cell’s repair mechanisms then modify the gene at that location.
Q2: What are the main applications of CRISPR technology today?
Ans: CRISPR is used in gene therapy for genetic diseases, cancer immunotherapy, agricultural crop improvement, and basic biological research.
Q3: What are the key limitations of CRISPR-Cas9?
Ans: Limitations include off-target effects, challenges in delivering the CRISPR system to target cells, ethical concerns about germline editing, and limited types of genetic modifications.
Q4: What are base editors and prime editors?
Ans: Base editors directly convert one DNA base to another without cutting DNA strands. Prime editors use a reverse transcriptase fused to Cas9 to perform versatile DNA edits without double-strand breaks.
Q5: How might genetic engineering impact agriculture in the future?
Ans: It will enable creation of crops that are more resilient to climate stress, require fewer chemicals, have higher yields, and are more nutritious, helping address food security and sustainability.
Similar Articles
Find more relatable content in similar Articles

The Future of Electric Planes ..
The aviation industry is under.. Read More

E-Waste Crisis: The Race to Bu..
The rapid growth of electronic.. Read More

Virtual Reality Therapy: Heali..
Virtual Reality Therapy (VRT) .. Read More

3D-Printed Organs: Are We Clos..
3D-printed organs are at the f.. Read More
Explore Other Categories
Explore many different categories of articles ranging from Gadgets to Security
Smart Devices, Gear & Innovations
Discover in-depth reviews, hands-on experiences, and expert insights on the newest gadgets—from smartphones to smartwatches, headphones, wearables, and everything in between. Stay ahead with the latest in tech gear
Apps That Power Your World
Explore essential mobile and desktop applications across all platforms. From productivity boosters to creative tools, we cover updates, recommendations, and how-tos to make your digital life easier and more efficient.
Tomorrow's Technology, Today's Insights
Dive into the world of emerging technologies, AI breakthroughs, space tech, robotics, and innovations shaping the future. Stay informed on what's next in the evolution of science and technology.
Protecting You in a Digital Age
Learn how to secure your data, protect your privacy, and understand the latest in online threats. We break down complex cybersecurity topics into practical advice for everyday users and professionals alike.
© 2025 Copyrights by rTechnology. All Rights Reserved.