
Bioinformatics for non-model organisms: tools, challenges, biodiversity.
Exploring bioinformatics for non-model organisms opens doors to understanding the vast genetic diversity of life beyond traditional lab species. By leveraging advanced sequencing, computational tools, and AI-driven analyses, researchers can study evolution, adaptation, and ecosystem dynamics, uncover unique genes, and support biodiversity conservation. Despite challenges, these approaches are transforming science and preserving Earth’s biological heritage.
✨ Raghav Jain
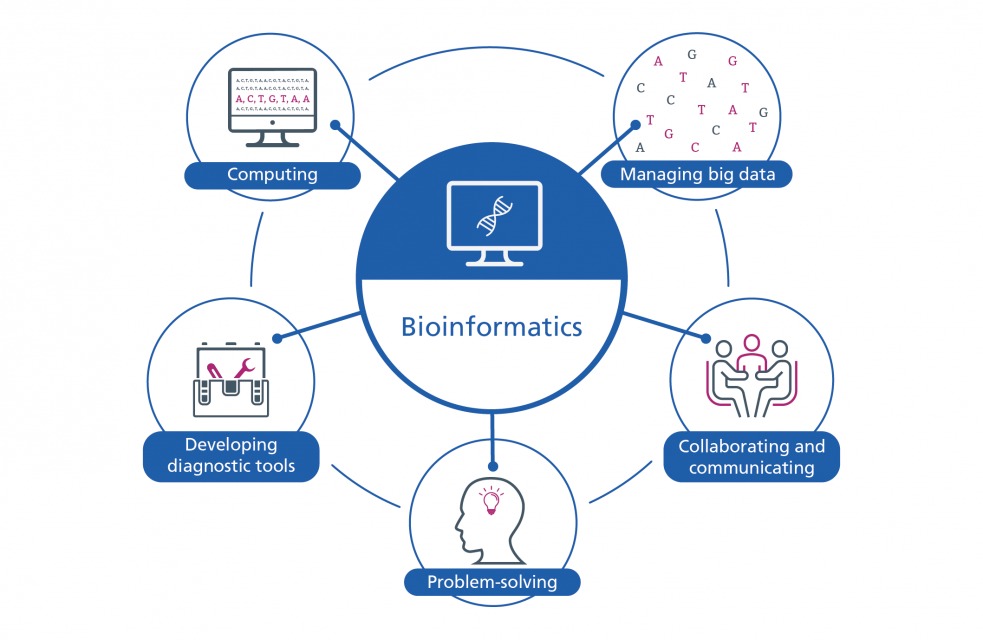
Introduction
In the genomic era, bioinformatics serves as the backbone of biological discovery. While model organisms like E. coli, Drosophila melanogaster, Mus musculus (mouse), and Arabidopsis thaliana have long dominated laboratory research due to their well-characterized genomes, the world teems with millions of species whose genetic blueprints remain largely unexplored. These are known as non-model organisms—species that lack comprehensive genomic resources, established research protocols, or standardized datasets.
Studying non-model organisms has immense value: it enhances our understanding of evolution, ecology, and biodiversity, offering fresh insights into gene functions, adaptive traits, and ecosystem dynamics. From extremophiles thriving in volcanic vents to rare medicinal plants in rainforests, non-model species hold untapped biological treasures. However, analyzing them through bioinformatics presents unique technical and conceptual challenges, from genome assembly difficulties to annotation errors and limited reference databases.
This article explores the tools, challenges, and biodiversity implications of bioinformatics research on non-model organisms—charting how modern computational innovations are helping scientists decode life beyond the lab-bred few.
1. Understanding Non-Model Organisms
Non-model organisms are any species not traditionally used as standardized systems in genetics, developmental biology, or physiology. Unlike model organisms, which have abundant genomic data and well-developed research methods, non-model species are often ecologically specialized, evolutionarily distinct, or rarely studied. Examples include coral reef organisms, wild relatives of crops, deep-sea microbes, or endangered mammals.
These species often possess unique biological adaptations—such as antifreeze proteins in Antarctic fish or desiccation resistance in desert plants—that cannot be understood solely by studying model organisms. Bioinformatics provides the computational infrastructure to analyze their genomes, transcriptomes, and proteomes, bridging the gap between ecological data and molecular biology.
2. Tools and Techniques for Non-Model Organism Bioinformatics
The explosion of next-generation sequencing (NGS) technologies has made it possible to sequence even complex genomes at reduced costs. However, working with non-model species often requires specialized tools and de novo approaches since reference genomes are absent or incomplete.
a. Genome Assembly Tools
Without a reference genome, scientists rely on de novo assembly to piece together short DNA fragments into complete genomes.
- SPAdes – ideal for microbial and small genome assembly.
- Canu – optimized for long-read data from PacBio or Oxford Nanopore technologies.
- SOAPdenovo2 – effective for assembling large eukaryotic genomes.
- Flye and MaSuRCA – combine long and short reads to improve accuracy.
These assemblers reconstruct genomic sequences by overlapping millions of reads, but the process is computationally demanding and error-prone when dealing with repetitive or highly heterozygous genomes.
b. Annotation Pipelines
Once a genome is assembled, identifying genes, regulatory regions, and functional elements becomes critical.
- MAKER and AUGUSTUS – for automated gene prediction.
- InterProScan and BLAST2GO – to annotate gene functions by comparison with known databases.
- BUSCO (Benchmarking Universal Single-Copy Orthologs) – assesses completeness of genome assemblies.
Annotation for non-model organisms is often limited by poor database representation, requiring researchers to use comparative genomics with related species or to rely on RNA-Seq data to identify expressed genes.
c. Transcriptomics and Proteomics Tools
When genomic data are scarce, researchers turn to transcriptome sequencing (RNA-Seq) or proteomics to study gene expression and protein diversity.
- Trinity – widely used for de novo transcriptome assembly.
- Salmon and Kallisto – for fast quantification of gene expression.
- Proteome Discoverer and MaxQuant – to identify and quantify proteins from mass spectrometry data.
These tools help unravel metabolic pathways, developmental processes, and responses to environmental stress in non-model species.
d. Phylogenomics and Population Genomics
Bioinformatics for non-model organisms also enables large-scale phylogenetic and population genetic studies.
- OrthoFinder – detects orthologous genes across species for evolutionary comparisons.
- STRUCTURE and ADMIXTURE – reveal population structure and gene flow.
- IQ-TREE and RAxML – construct evolutionary trees using genomic alignments.
- VCFtools and ANGSD – handle variant calling and diversity analysis.
These analyses deepen our understanding of adaptation, speciation, and genetic drift across ecosystems.
3. Challenges in Bioinformatics for Non-Model Species
While the tools exist, working with non-model organisms presents unique biological, computational, and infrastructural challenges.
a. Data Limitations
Non-model organisms often lack reference genomes, curated gene annotations, or baseline molecular data. This leads to difficulties in validating assemblies, identifying homologous genes, or detecting errors.
b. Genome Complexity
Many non-model organisms possess large, repetitive, polyploid, or highly heterozygous genomes, complicating assembly. For instance, many plants have multiple genome copies due to hybridization or polyploidy, while some amphibians or insects have repetitive DNA that confuses assembly algorithms.
c. Lack of Standardized Pipelines
Unlike model species (where databases like Ensembl or FlyBase exist), there are few standardized pipelines for analyzing non-model data. Researchers must customize workflows, leading to inconsistencies in results and difficulty reproducing studies.
d. Computational Demands
De novo assembly, annotation, and downstream analyses require powerful computing infrastructure, often beyond the reach of small laboratories or conservation teams.
e. Database Bias and Annotation Errors
Functional annotation tools rely on homology searches against databases dominated by model species. As a result, many genes in non-model organisms remain labeled as “hypothetical proteins,” or worse, misannotated due to poor matches.
f. Funding and Resource Constraints
Most funding agencies prioritize model organism research, leaving non-model species—often those critical to ecosystem health or conservation—understudied. This limits both data generation and maintenance of open-access genomic resources.
4. Opportunities and Innovations
Despite these challenges, the field is rapidly evolving with new methods to bridge the information gap.
a. Long-Read and Hybrid Sequencing
Technologies like PacBio HiFi and Oxford Nanopore provide long DNA reads that span repetitive regions, improving assembly accuracy. Combined with Illumina short reads, hybrid assemblies are becoming the gold standard for non-model genomes.
b. Pangenomics and Reference Graphs
Instead of a single reference genome, pangenomes capture genetic diversity within species. This approach benefits wild relatives of crops or geographically dispersed populations, highlighting adaptive variations.
c. Cloud-Based Bioinformatics
Platforms like Galaxy, Cyborg, Terra, and NCBI’s AWS Cloud now offer user-friendly interfaces and scalable computing power, enabling global collaboration even for resource-limited labs.
d. Machine Learning and AI
AI-driven approaches can predict gene functions, classify protein families, and detect adaptive mutations without relying solely on existing annotations. This is especially transformative for novel species.
e. Citizen and Conservation Genomics
Portable sequencers like Oxford Nanopore’s MinION allow field researchers and conservationists to perform on-site DNA sequencing. Combined with open-access databases (e.g., NCBI SRA, GenBank), this democratizes biodiversity genomics.
5. Bioinformatics and Biodiversity Conservation
The implications of non-model organism bioinformatics extend far beyond the laboratory. Biodiversity genomics—powered by computational biology—can revolutionize conservation, agriculture, and ecosystem management.
a. Conservation Genomics
By decoding the genomes of endangered species, scientists can detect inbreeding, loss of genetic diversity, or adaptive genes critical for survival. Initiatives like the Earth BioGenome Project aim to sequence all eukaryotic life, offering unprecedented tools for global conservation.
b. Climate Adaptation Studies
Non-model organisms adapted to extreme environments, such as heat-tolerant corals or drought-resistant plants, provide insights into climate resilience mechanisms. Bioinformatics allows identification of key stress-response genes that could aid agriculture or restoration efforts.
c. Microbiome and Ecosystem Health
Environmental DNA (eDNA) and metagenomics enable the study of microbial communities that sustain ecosystems. Non-model microbes often drive nutrient cycling, carbon fixation, or symbiotic relationships vital to biodiversity.
d. Agricultural Innovation
Wild relatives of crops harbor genes conferring resistance to pests, diseases, and stress. Sequencing their genomes allows breeders to reintroduce these traits into cultivated varieties through genomic-assisted breeding.
e. Cultural and Ethical Dimensions
Indigenous and local communities often live in biodiversity-rich regions. Integrating ethical genomics frameworks ensures equitable access, data sovereignty, and respect for traditional knowledge linked to biological resources.
6. Case Studies
a. Coral Genomics
Coral reefs are biodiversity hotspots under threat from ocean warming. Bioinformatic analyses of coral genomes and their symbiotic algae (Symbiodinium) have revealed genes linked to heat tolerance, aiding reef restoration efforts.
b. Axolotl Regeneration
The axolotl (Ambystoma mexicanum), a non-model amphibian, has a 32-billion-base-pair genome. Its bioinformatic decoding has uncovered genes responsible for limb regeneration, potentially revolutionizing regenerative medicine.
c. Wild Banana and Rice Genomics
Sequencing wild relatives of bananas (Musa acuminata) and rice (Oryza rufipogon) identified disease resistance genes absent in domesticated crops, guiding sustainable agriculture.
7. Future Directions
The future of bioinformatics for non-model organisms lies in integration, accessibility, and inclusivity. Multi-omics (combining genomics, transcriptomics, metabolomics, and epigenomics) will reveal complex biological networks. Global collaborations and open databases will ensure that data from every species, no matter how obscure, contribute to understanding life’s full complexity.
Efforts like Genome 10K, B10K (Bird Genomes Project), and Earth BioGenome Project exemplify the ambition to catalog all genomic diversity. With AI-driven annotation, cloud computing, and portable sequencing, studying non-model organisms is no longer a privilege—it’s becoming a global scientific movement.
Bioinformatics, the fusion of biology, computer science, and statistics, has evolved into an indispensable discipline for exploring life at the molecular level. Traditionally, the field has revolved around “model organisms” such as E. coli, Drosophila melanogaster, Arabidopsis thaliana, and Mus musculus, which possess well-documented genetic data, standardized laboratory methods, and global research communities supporting their study. However, the real biological world extends far beyond these select few species. The vast majority of living organisms—ranging from deep-sea microbes to endangered mammals and rare plants—fall into the category of non-model organisms, which are species lacking extensive genomic information or established experimental protocols. The study of these non-model species is vital for understanding the full spectrum of life’s diversity, evolution, and ecological adaptation. Bioinformatics has become the key enabler for such studies, offering computational methods to decode genomes, analyze gene expression, and infer evolutionary relationships where laboratory data are scarce or incomplete. Yet, applying bioinformatics to non-model organisms brings unique complexities, including difficulties in assembling large or repetitive genomes, annotating unknown genes, and managing data without standardized references. The technological revolution in sequencing—particularly next-generation and long-read platforms—has made genome analysis cheaper and faster, allowing researchers to venture beyond the boundaries of conventional species. De novo assembly tools like SPAdes, Canu, Flye, and MaSuRCA have empowered scientists to construct genomes from scratch without relying on existing templates, while annotation pipelines such as MAKER, AUGUSTUS, InterProScan, and BUSCO facilitate gene prediction and functional characterization even in poorly studied organisms. Transcriptome assemblers like Trinity and quantification tools such as Kallisto and Salmon have made it possible to study expression profiles in species where genomic resources are limited. Population genomic tools like STRUCTURE, VCFtools, and ANGSD help in analyzing genetic diversity and adaptation patterns, while phylogenetic software such as IQ-TREE and RAxML supports evolutionary analysis across taxa. Despite these advances, several challenges remain central to non-model organism bioinformatics. Many non-model species possess highly repetitive, polyploid, or heterozygous genomes, making assembly error-prone and computationally intensive. Annotation accuracy is another major hurdle because existing protein and gene databases are biased toward model species, leading to thousands of “hypothetical proteins” and misannotated sequences in newly sequenced genomes. The absence of standardized pipelines also makes cross-study comparisons difficult, as researchers often modify or create custom workflows tailored to their species of interest. Moreover, de novo assembly and comparative analyses require powerful computational resources, often inaccessible to smaller laboratories or conservation groups in biodiversity-rich but resource-limited regions. Funding priorities add another layer of difficulty; governments and institutions tend to invest in research with immediate economic or medical implications, leaving many ecologically important non-model species underrepresented. Yet, despite these obstacles, the momentum in this field continues to grow due to the development of innovative technologies and open-access platforms. Long-read sequencing methods such as PacBio HiFi and Oxford Nanopore produce lengthy, high-quality DNA reads capable of resolving repetitive regions and structural variants, dramatically improving assembly accuracy for complex genomes. The concept of pangenomics, which constructs reference graphs capturing the full genetic diversity within a species or genus, is especially valuable for wild crop relatives and ecologically diverse populations. In addition, cloud-based bioinformatics environments like Galaxy, Terra, and NCBI’s AWS Cloud have democratized access to high-performance computing, enabling global collaboration and reproducibility. The application of machine learning and AI in functional genomics has opened new frontiers, allowing scientists to predict gene function and adaptive traits in the absence of homologous data. These computational innovations are now being integrated into citizen science and conservation genomics through portable devices such as the Oxford Nanopore MinION, which allows real-time DNA sequencing directly in the field. Such tools are invaluable for remote biodiversity assessments, enabling rapid identification of species, tracking of illegal wildlife trade, or monitoring genetic diversity in threatened populations. The implications of this work are far-reaching, particularly in the realm of biodiversity conservation. Genomic data from non-model organisms provide critical insights into the health of endangered species, revealing inbreeding patterns, adaptive gene variants, and evolutionary potential under changing environmental conditions. For instance, the Earth BioGenome Project aims to sequence all known eukaryotic species, representing one of the most ambitious biodiversity initiatives in human history. Studies on non-model organisms such as coral reefs and their symbionts have identified heat-tolerance genes that could guide restoration of climate-affected reefs. Similarly, the sequencing of axolotl genomes has revealed regeneration-related genes with potential medical applications, while analysis of wild rice and banana species has uncovered disease-resistance genes useful for crop improvement. Beyond applied science, non-model organism bioinformatics enhances our understanding of ecological balance and evolution, showing how species adapt, diverge, and coexist across environments. The study of microbial communities through metagenomics and environmental DNA (eDNA) further expands this vision, uncovering unseen biodiversity in soil, water, and even the atmosphere. Each new dataset contributes to a more inclusive view of life, illuminating connections between genotype, phenotype, and ecosystem dynamics. However, as data collection expands, ethical and cultural considerations must also guide the field—ensuring that genomic information from biodiverse regions respects data sovereignty, indigenous rights, and fair benefit sharing. Looking ahead, the integration of multi-omics (genomics, transcriptomics, proteomics, metabolomics, and epigenomics) will yield an even deeper understanding of organismal complexity, while AI-enhanced predictive models will enable scientists to simulate evolutionary outcomes or environmental responses. In conclusion, bioinformatics for non-model organisms stands at the frontier of modern biology—a domain of vast potential and continuing challenges. It bridges molecular and ecological sciences, linking the digital code of DNA to the living reality of Earth’s diverse species. Though constrained by limited data and resources, it thrives on innovation, collaboration, and curiosity. As sequencing costs drop and analytical tools become more accessible, the genetic study of non-model species will no longer be an exception but the norm. This paradigm shift not only expands scientific knowledge but also reinforces humanity’s responsibility to protect and celebrate the full spectrum of life on Earth—each genome, no matter how obscure, contributing a vital note to the symphony of biodiversity.
Bioinformatics for non-model organisms represents one of the most exciting frontiers in modern biology, bridging the gap between the wealth of knowledge accumulated from traditional model species and the vast, largely unexplored diversity of life on Earth, and its significance cannot be overstated, because while model organisms like E. coli, Drosophila melanogaster, Mus musculus, and Arabidopsis thaliana have provided foundational insights into genetics, molecular biology, and development, they account for only a tiny fraction of the world’s biodiversity, leaving millions of species with unique adaptations, ecological roles, and evolutionary histories almost entirely uncharacterized, and this knowledge gap presents both a challenge and an opportunity for the field of bioinformatics, which has the computational tools, algorithms, and data structures capable of processing massive amounts of genetic, transcriptomic, and proteomic information even when reference genomes are lacking, as is often the case with non-model species, because many of these organisms do not have well-established laboratory protocols, curated databases, or standardized annotations, and their genomes are frequently complex, large, polyploid, or highly repetitive, making de novo assembly a computationally intensive and technically demanding process that requires specialized algorithms and hybrid sequencing strategies combining short reads from platforms like Illumina with long reads from PacBio or Oxford Nanopore to resolve repetitive elements, structural variants, and complex regions, and once assembled, the annotation of these genomes presents another layer of difficulty because existing reference databases are heavily biased toward model organisms, meaning that many predicted genes in non-model species are often labeled as “hypothetical proteins” or misannotated, necessitating advanced tools such as MAKER, AUGUSTUS, InterProScan, and BUSCO for functional prediction, while RNA-Seq data can provide transcript evidence to support gene models, yet even these approaches require careful experimental design, quality control, and computational resources to generate meaningful results, and beyond genome assembly and annotation, bioinformatics enables the analysis of gene expression patterns, metabolic pathways, and adaptive responses in non-model organisms through transcriptomics and proteomics, with tools like Trinity, Salmon, and Kallisto for transcriptome assembly and quantification, as well as MaxQuant or Proteome Discoverer for protein identification, all of which allow researchers to understand how these organisms respond to environmental pressures, pathogens, or climate change, while population genomics and phylogenomics further expand the potential of bioinformatics in non-model research, employing software such as STRUCTURE, ADMIXTURE, VCFtools, ANGSD, IQ-TREE, and RAxML to infer population structure, genetic diversity, and evolutionary relationships, revealing patterns of adaptation, speciation, and gene flow that are often invisible in model organisms, and despite the technological progress, several challenges persist, including the lack of standardized workflows, insufficient funding, and computational bottlenecks, as many non-model species are studied in labs or field stations that may not have access to high-performance computing clusters, cloud-based solutions like Galaxy, Terra, or NCBI’s AWS platforms are helping to democratize access, allowing researchers worldwide to perform complex analyses, and the integration of AI and machine learning into genome annotation and functional prediction is another rapidly growing trend, enabling the identification of genes, regulatory elements, and adaptive features without reliance solely on homology-based methods, which is particularly useful for species with no close relatives in existing databases, and the impact of these developments extends far beyond basic research, with significant implications for conservation biology, ecosystem management, and agriculture, as genomic data from non-model organisms allow scientists to monitor genetic diversity in endangered populations, identify adaptive traits critical for survival under climate change, track invasive species, and inform restoration strategies, exemplified by projects like the Earth BioGenome Project, Genome 10K, and B10K, which aim to sequence tens of thousands of species to capture the full breadth of eukaryotic diversity, and in practical terms, studying non-model species has already produced transformative insights, such as the discovery of heat-tolerant genes in coral symbionts that may help reefs survive ocean warming, or the elucidation of regeneration-related genes in the axolotl, which holds promise for regenerative medicine, while the genomes of wild relatives of crops like rice and bananas have revealed resistance genes that could enhance food security, and environmental DNA (eDNA) and metagenomic approaches further extend the reach of bioinformatics, enabling the detection and monitoring of microbial communities and rare species without direct observation, which is particularly valuable in inaccessible or fragile ecosystems, and portable sequencing technologies like the Oxford Nanopore MinION have made it possible to perform field-based sequencing, empowering citizen scientists, conservationists, and researchers in remote regions to contribute to biodiversity monitoring and genomic research, thereby creating a more inclusive and collaborative scientific ecosystem, yet these advances also bring ethical and cultural responsibilities, particularly regarding the collection, use, and sharing of genomic data from regions inhabited by indigenous and local communities, emphasizing the need for equitable access, benefit sharing, and respect for traditional knowledge, and looking ahead, the future of bioinformatics for non-model organisms lies in integrating multi-omics data—combining genomics, transcriptomics, proteomics, metabolomics, and epigenomics—to gain a holistic understanding of organismal biology, while AI-driven predictive models could simulate environmental responses, evolutionary trajectories, or gene-environment interactions, ultimately enabling science to anticipate and mitigate the impacts of climate change, habitat loss, and human activities on biodiversity, and by continuing to expand sequencing coverage, improve analytical pipelines, and foster global collaboration, bioinformatics can transform our understanding of life on Earth, revealing the hidden complexity of species that have long been neglected, and underscoring the profound realization that every organism, no matter how obscure, plays a crucial role in the web of life, making the study of non-model species not merely a scientific endeavor but a vital component of conservation, sustainable development, and the preservation of planetary biodiversity for future generations, as it highlights the interconnectedness of ecosystems, the adaptive ingenuity of organisms, and the critical need to protect the genetic heritage of our planet while leveraging computational tools to unlock the secrets encoded within DNA, ultimately demonstrating that bioinformatics is not just a method for analyzing data but a transformative approach that enables humanity to explore, understand, and safeguard the extraordinary diversity of life that sustains our world.
Conclusion
Bioinformatics for non-model organisms is transforming biology, ecology, and conservation. Through powerful computational tools, researchers can decode genomes once considered too complex or inaccessible, revealing the secrets of life in its most diverse forms. Although challenges remain—such as limited data, computational constraints, and annotation bias—technological innovations are rapidly narrowing these gaps.
Non-model organisms represent the majority of Earth’s biodiversity, yet their genomic data remain the least understood. By extending bioinformatics beyond traditional model species, science moves closer to a holistic understanding of evolution, adaptation, and ecosystem resilience. Ultimately, the study of non-model organisms not only enriches biology but also strengthens humanity’s ability to protect and sustain life on Earth.
Q&A Section
Q1 :- What are non-model organisms?
Ans :- Non-model organisms are species that lack extensive genetic resources or established research systems, unlike model organisms such as E. coli or Drosophila. They include wild, rare, or ecologically unique species studied using emerging bioinformatics tools.
Q2 :- Why are non-model organisms important in research?
Ans :- They provide insights into evolutionary adaptation, biodiversity, and ecological resilience. Many non-model species possess unique genes or traits valuable for medicine, agriculture, and climate adaptation.
Q3 :- What are the main challenges in studying non-model organisms?
Ans :- The major challenges include lack of reference genomes, computational limitations, complex genome structures, annotation errors, and limited funding or standardized workflows.
Q4 :- Which tools are used for genome assembly in non-model species?
Ans :- Tools like SPAdes, Canu, Flye, and MaSuRCA are widely used for de novo genome assembly, often combining short and long sequencing reads for accuracy.
Q5 :- How does bioinformatics support biodiversity conservation?
Ans :- Genomic data help identify endangered populations, track genetic diversity, detect adaptive traits, and inform restoration strategies, enabling more effective conservation planning.
Similar Articles
Find more relatable content in similar Articles

Data Centers and the Planet: M..
As cloud computing becomes the.. Read More
Wearable Health Sensors: The D..
Wearable health sensors are re.. Read More

Digital DNA: The Ethics of Gen..
Digital DNA—the digitization a.. Read More

Protecting Kids in the Digital..
In an increasingly connected w.. Read More
Explore Other Categories
Explore many different categories of articles ranging from Gadgets to Security
Smart Devices, Gear & Innovations
Discover in-depth reviews, hands-on experiences, and expert insights on the newest gadgets—from smartphones to smartwatches, headphones, wearables, and everything in between. Stay ahead with the latest in tech gear
Apps That Power Your World
Explore essential mobile and desktop applications across all platforms. From productivity boosters to creative tools, we cover updates, recommendations, and how-tos to make your digital life easier and more efficient.
Tomorrow's Technology, Today's Insights
Dive into the world of emerging technologies, AI breakthroughs, space tech, robotics, and innovations shaping the future. Stay informed on what's next in the evolution of science and technology.
Protecting You in a Digital Age
Learn how to secure your data, protect your privacy, and understand the latest in online threats. We break down complex cybersecurity topics into practical advice for everyday users and professionals alike.
© 2025 Copyrights by rTechnology. All Rights Reserved.